It is 25 years since the U.N. called on tobacco companies to make every effort to reduce the toxicity of tobacco products. A lot has happened since then. PMI took the call seriously and has led the way in tobacco harm reduction, investing billions into pioneering a portfolio of less harmful alternative products to conventional cigarettes. In 2016, we announced our transformative goal: To deliver a smoke-free future by focusing our resources on developing, scientifically substantiating, and responsibly commercializing smoke-free products that are less harmful than continued smoking. Our goal is that these products, which we call reduced-risk products (RRPs)*, will replace cigarettes as soon as possible for those adults who would otherwise continue smoking.
The theory of tobacco harm reduction
People have always taken part in activities with a certain degree of danger, despite knowledge of the potential harm involved. For example, when people first started driving, it was more dangerous. Over time, the automotive industry spearheaded safety technologies, while regulators introduced road safety legislation that reduced the risks. This approach—aimed at eliminating or reducing as much as possible the negative effects rather than the activity itself—is the essence of harm reduction.
The same idea applies to smoking. At PMI, we are clear that the best choice is always to quit tobacco and nicotine altogether. But it is estimated that over one billion smokers continue to use cigarettes worldwide.
It is important to continue encouraging them to stop and, in fact, not to start in the first place. But for those who continue to smoke, an innovative approach is needed—one that includes offering better alternatives such as e-cigarettes and heated tobacco products, which could help adult smokers to leave cigarettes behind. This is the concept behind tobacco harm reduction.
Reducing harm doesn’t only apply to individuals. It can impact society on a much larger scale, because if millions of adults who would otherwise continue smoking switch to a science-backed smoke-free alternative and stop smoking, the entire population could benefit. This is because smoking is the most harmful form of tobacco consumption.
At PMI, we have committed to accelerating the end of smoking by offering adults better alternatives to cigarettes. Evidence from markets where these products have been launched suggests that they can cause a dramatic decline in cigarette sales.
The science of tobacco harm reduction
To demonstrate that switching to our RRPs results in a significant reduction in the risk of harm compared with continued smoking, we are following a rigorous scientific assessment program. This program applies well-recognized practices in toxicology, as well as an innovative Systems Toxicology-based approach to risk assessment.
Our program is in line with the draft guidance from the U.S. Food and Drug Administration for a Modified Risk Tobacco Product Application (MRTPA). We conduct our research in accordance with international standards and practices, such as the internationally accepted Good Laboratory Practices (GLPs) and Good Clinical Practices (GCPs).
Our assessment program covers the full spectrum of activities, from initial product development to the monitoring of these products once they are on the market.
Are PMI’s smoke-free products less harmful than cigarettes?
Decades of epidemiological data have demonstrated that the development of smoking-related diseases is triggered by the inhalation of harmful and potentially harmful chemicals (HPHCs) found in cigarette smoke.
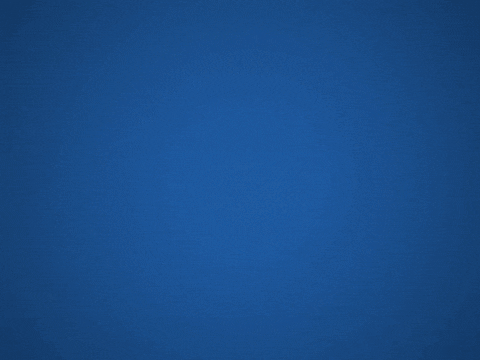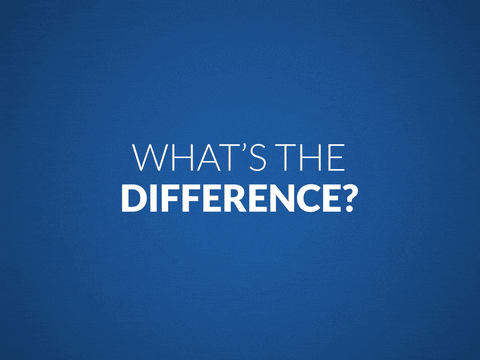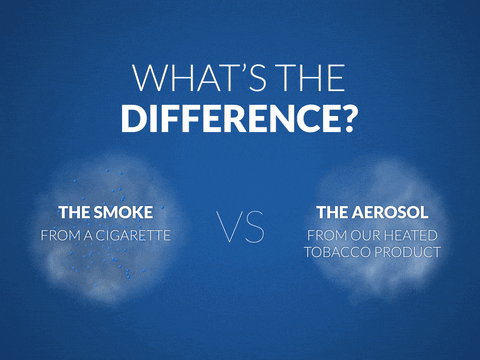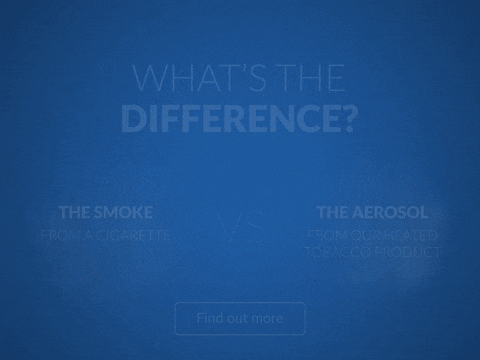
When a cigarette is lit, the tobacco burns and creates smoke, which contains more than 6,000 chemicals. Public health authorities have classified approximately 100 of them as causes or potential causes of smoking-related diseases such as lung cancer, cardiovascular disease, and emphysema.
It’s hard to speak in general terms about the safety of an entire category of smoke-free products, which includes various types of products, including heated tobacco products and e-cigarettes. But if a product does not burn tobacco, it is likely to produce lower levels of harmful chemicals than a cigarette. It’s therefore likely to be a better choice than continued smoking.
Still, each product needs to be scientifically studied to assess its potential to reduce risk. If it does this to a significant degree, it means the product is likely to present a reduced risk of harm compared to cigarettes.
Our findings demonstrate that the aerosol produced by our leading heated tobacco product contains on average 95 percent lower levels of harmful and potentially harmful constituents (HPHCs) than a cigarette. This does not mean they are risk-free, of course.
Unlike heated tobacco products, e-cigarettes do not contain tobacco leaves, and generally use a flavored liquid to deliver a nicotine vapor, which should substantially reduce exposure to HPHCs compared to cigarettes. Again, they are not risk-free.
Both heated tobacco products and e-cigarettes provide nicotine, which is addictive and not risk-free. However, nicotine is not the primary cause of smoking-related diseases.
Smoke-free alternatives are only for adults who would otherwise continue smoking. The best choice any smoker can make is to quit tobacco and nicotine altogether.
PMI’s smoke-free product assessment program
- Developing the product
Assessment of an RRP’s risk-reduction potential relies on the quality of the initial product design, and on strict manufacturing controls to ensure that the product delivers a consistent aerosol. We eliminate combustion and ensure that the product delivers fewer HPHCs. In this initial phase of designing a product, we verify that the product’s design does not pose any additional risks to those already known for combustible cigarettes. Only then can we begin to conduct further research. - Laboratory studies
Our next step is to verify the potential of an RRP’s aerosol to reduce risk compared to cigarette smoke by measuring a reduction in toxicity, as well as a reduction in risk, using laboratory models. If they are significantly reduced, we move on to clinical studies. - Clinical studies
Once we have completed our laboratory research, we conduct clinical studies with adult smokers to understand whether switching to RRPs reduces their exposure to toxic compounds. We also determine whether this leads to a favorable change in clinical risk markers associated with smoking-related diseases. The effects measured in adult smokers who switch to an RRP are compared with those in smokers who continue to smoke cigarettes, and with smokers who quit for the duration of the study. - Consumer use research
We conduct several types of perception and behavioral studies to better understand an RRP’s potential to benefit public health. These include research into how adult smokers perceive a product’s risk, and how they adopt and use an RRP under real-life conditions. We also verify that people who have never smoked, and former smokers, understand that RRPs are not intended for them. - Long-term assessment
Lastly, we monitor and research the use of our smoke-free alternatives to cigarettes once they are on the market to assess the product’s contribution to tobacco harm reduction.
In 1997, the U.N. Focal Point on Tobacco or Health recommended that in order to reduce the harm associated with tobacco use “[…] every effort should be made to reduce the toxicity of existing tobacco products.”
The U.N. set us the challenge of achieving tobacco harm reduction all those years ago.
We have risen to it by researching, assessing, and commercializing science-backed alternatives to continued smoking that have demonstrated a potential to reduce harm.
This breakthrough has presented our society with the opportunity of dramatically accelerating the decline of cigarette sales. Indeed, it is already happening in many countries. We have a vision for a smoke-free future, and we’re committed to making that vision a reality.
* Reduced-risk products (“RRPs”) is the term PMI uses to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing smoking. PMI has a range of RRPs in various stages of development, scientific assessment and commercialization. PMI’s RRPs are smoke-free products that contain and/or generate far lower quantities of harmful and potentially harmful constituents than found in cigarette smoke.