What do PMI scientists do?
This pioneering group of PMI scientists and technicians work collaboratively to create reduced-risk products that mimic the sensory components of cigarettes, without smoke. But what exactly is involved in the scientific process of driving a smoke-free product’s journey from conception to mass production?
It begins with the initial assessment of a product’s risk-reduction potential. This is based on the quality of the product’s design and on strict manufacturing quality controls to ensure it delivers a consistent aerosol. During this early phase, tests are conducted by PMI scientists to determine if the product design will lead to an overall and significant reduction in harmful and potentially harmful constituents (HPHCs) in the aerosol, compared with cigarette smoke.

It’s time to make smoking history
Voiceover: Jacek Olczak, CEO, Philip Morris International
This company wants to change the world.
If we can get there this is going to be really phenomenal
Fast-paced music starts
Words on screen read: That’s why at Philip Morris International, we’ve committed to deliver a smoke-free future.
Voiceover: Stefano Volpetti, President, Smoke-Free Inhaled Products & Chief Consumer Officer, PMI.
This company has had the courage, the vision and leadership to establish a new direction.
Voiceover: Bin Li, Chief Product Officer, PMI
It's very difficult for a company to sign up for a big transformation like this.
Words on screen read: Our ambition? To move away from cigarettes.
Voiceover: Stacey Kennedy, President Americas Region and CEO of PMI’s U.S. business:
We are building our company's future around better alternatives to smoking.
Voiceover: Michael Voegele, Chief Digital and Information Officer, PMI:
I think it's a unique challenge being part of a team that revolutionizes the industry
Words on screen read: Since 2008, we’ve invested over 10.5 billion dollars in scientific substantiated smoke-free products.
Voiceover: Gregoire Verdeaux, Senior Vice President, External Affairs, PMI:
The end of cigarettes is doable and it is about setting a date, setting a timetable and setting a path to go there.
Words on screen read: And by 2030, we aim to have more than two-thirds of our net revenue come from smoke-free products.
In 2023, we’re at 36.2%.
Voiceover: Marian Salzman, Senior Vice President & Chief Corporate Citizenship Officer, PMI:
I think we've become part of the ongoing dialogue around companies that can change
Voiceover: Lars Dahlgren, President, Smoke-Free Oral Products and CEO, Swedish Match:
I've been impressed with the boldness of PMI articulating that the future is smoke free products.
Words on screen read: Currently our smoke-free products are available in 82 markets
Voiceover: Dr Moira Gilchrist, Chief Communications Officer, PMI:
We set out to develop these products and gather scientific evidence.
Voiceover: Dr Badrul Chowdhury, Chief Life Sciences Officer, Smoke-Free Products:
You actually have a product where the hundred or so chemicals which are known to be harmful are reduced or eliminated. That's the scientific breakthrough.
Voiceover: Dr Moira Gilchrist, Chief Communications Officer, PMI:
This is transforming the entire industry
Voiceover: Jacek Olczak, CEO, Philip Morris International:
The evidence is clear. Smoke-free alternatives can accelerate the end of smoking. The world has to move now to help smokers to move to the better alternative.
Words on screen read: 27.4 million adults globally are using our leading heated tobacco product, of which 19.7 million have switched completely and stopped smoking.
Voiceover: Jacek Olczak, CEO, Philip Morris International:
It's time to make smoking history.
Philip Morris International is seen on screen.
Measuring toxicity
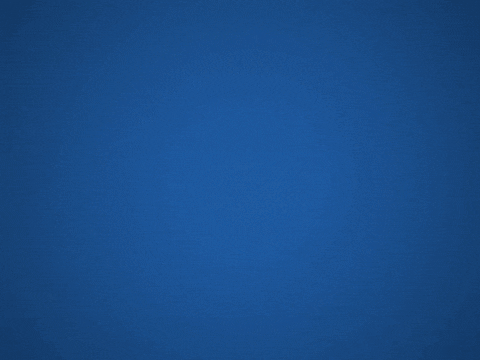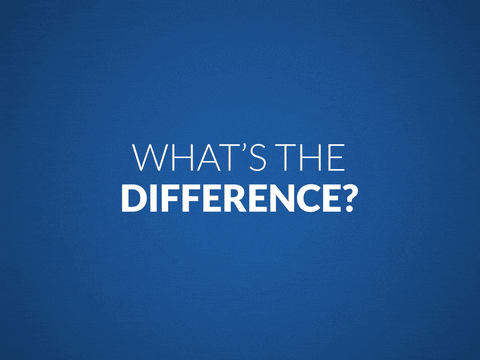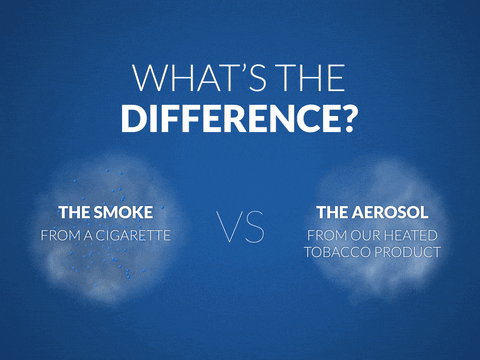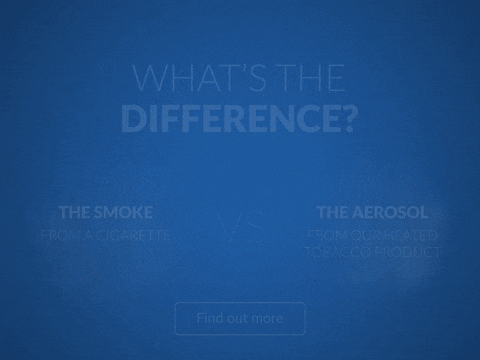
Another area of research involves measuring the level of toxicity in the aerosol of smoking alternatives compared to cigarette smoke.
PMI carries out these assessments using standard and advanced toxicology methods (comparable to those used in the pharmaceutical industry) to establish whether the reduction in HPHCs leads to a lower impact of the product aerosol on the biological
mechanisms underlying smoking-related diseases.
Next up is the clinical assessment stage, in which PMI scientists study adult smokers to determine if switching to the better alternative to smoking reduces their exposure to harmful compounds.
The effects of using the smoke-free product are assessed against both people who have continued smoking cigarettes and those who have quit tobacco and nicotine completely.
Perception and behavior studies
Then, PMI scientists conduct wider perception and behavior studies to assess a smoke-free product’s potential to benefit public health.
A key area of this is understanding how different groups of people view the risk profile of a smoke-free
product and how likely they are to permanently adopt it instead of smoking cigarettes, while making every effort to ensure that the product does not appeal to non-smokers, former smokers, or those trying to quit nicotine and tobacco products completely.
Long-term assessment
Finally, there’s the long-term assessment stage. This involves monitoring and researching the use of PMI’s smoke-free products once they’re available to buy, in order to assess their contribution to harm reduction. This is achieved by collating qualitative and quantitative data on the use of the smoking alternative in the real world.
At the heart of each phase of product development and testing are the PMI scientists, all of whom are determined to reshape the future of public health.
PMI’s R&D employees are working with painstaking precision and ambition to make a difference to the lives of smokers across the world.
Jean-Claude Schneider, Global Head of Product Development Programs, said: “It’s more than a big shift. It’s a revolution. It’s a complete redesign of the organization towards this new objective, which is a smoke-free future.”
Share this article