For 150 years, the name “Philip Morris” was widely associated with cigarettes.
But in 2016, we committed to delivering a smoke-free future, disrupting our business—and our industry—from within.
Since then, we’ve focused on developing, scientifically substantiating and responsibly commercializing smoke-free products that are less harmful than smoking, with the aim of completely replacing cigarettes as soon as possible.
Our smoke-free alternatives to cigarettes do not burn tobacco or create smoke, and therefore generate significantly lower levels of toxic substances.
The totality of our studies indicates that, while not risk-free, switching completely to these smoke-free products is likely to present less risk of harm than continued smoking.

An estimated 42 million+ legal-age adults* are now using our smoke-free products , which we sell in 106 markets*.
*As of December 31, 2025. Important note: This information should be read in conjunction with the Philip Morris International Inc. earnings release dated February 6, 2026, as well as the accompanying glossary of key terms, definitions, explanatory notes, select financial information and reconciliations of non-GAAP financial measures, both of which are available on our Investor Relations page. “PMI,” “We,” “Our,” and “Us” refers to the entire Philip Morris International family of companies..
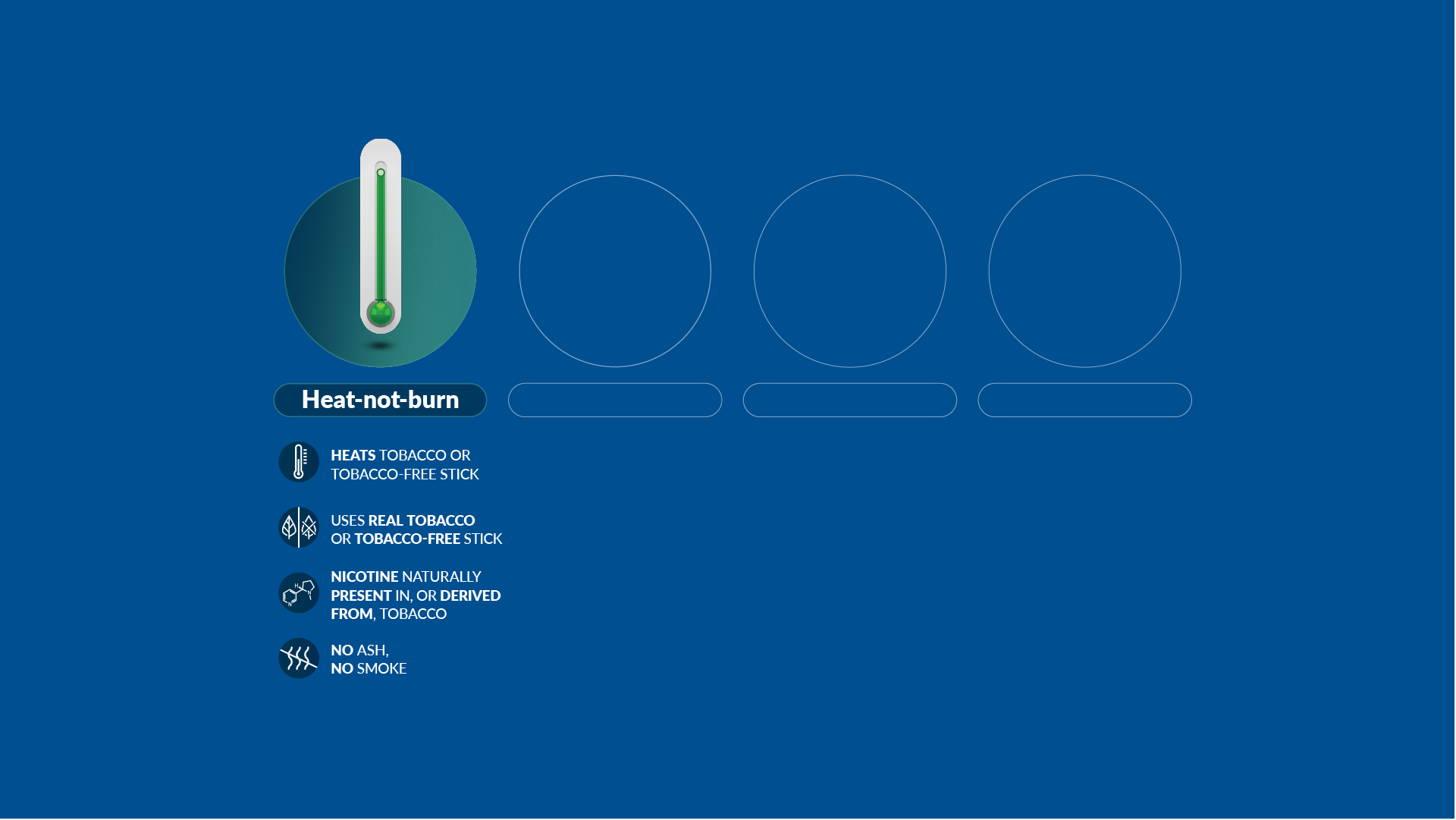
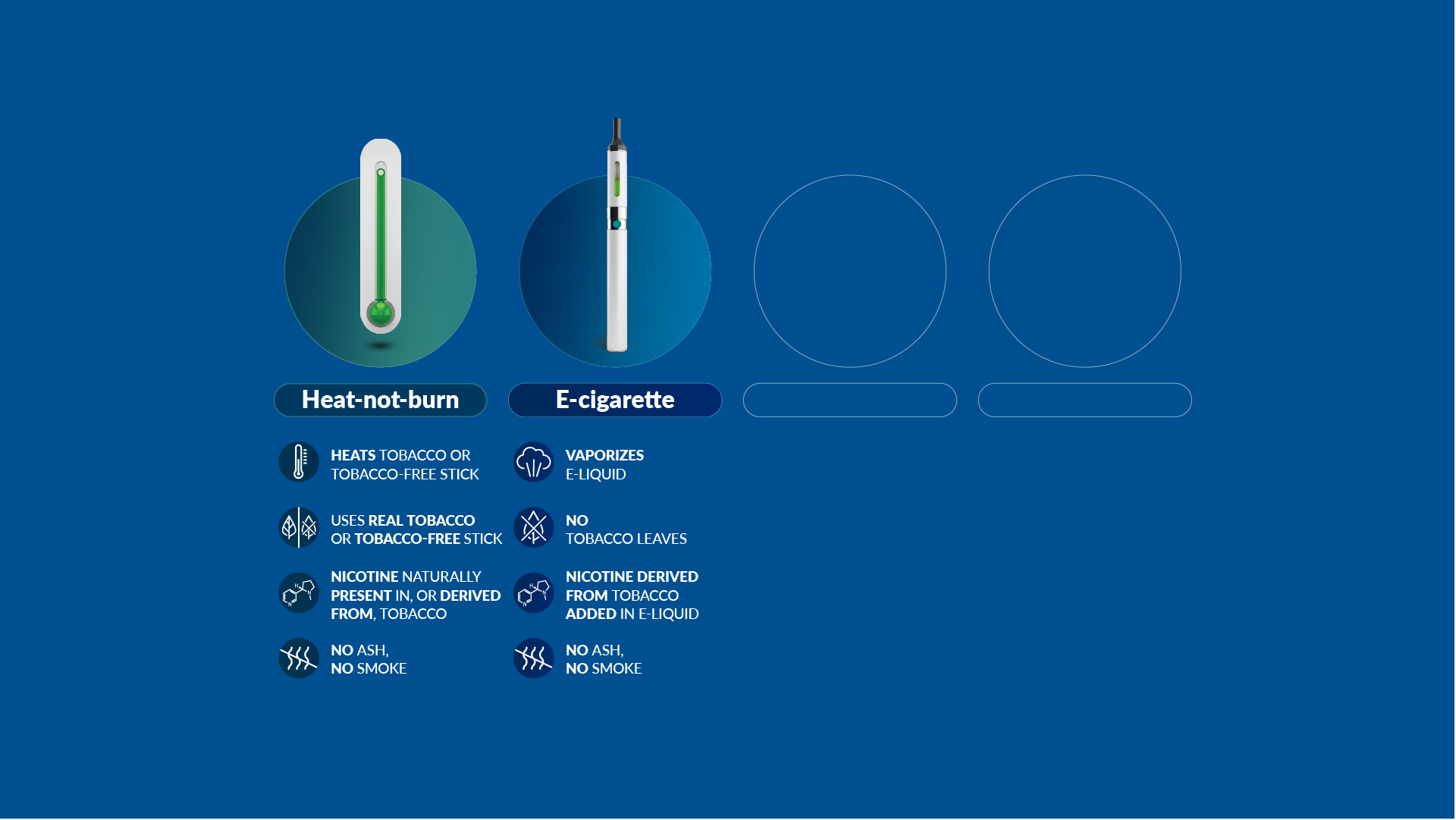
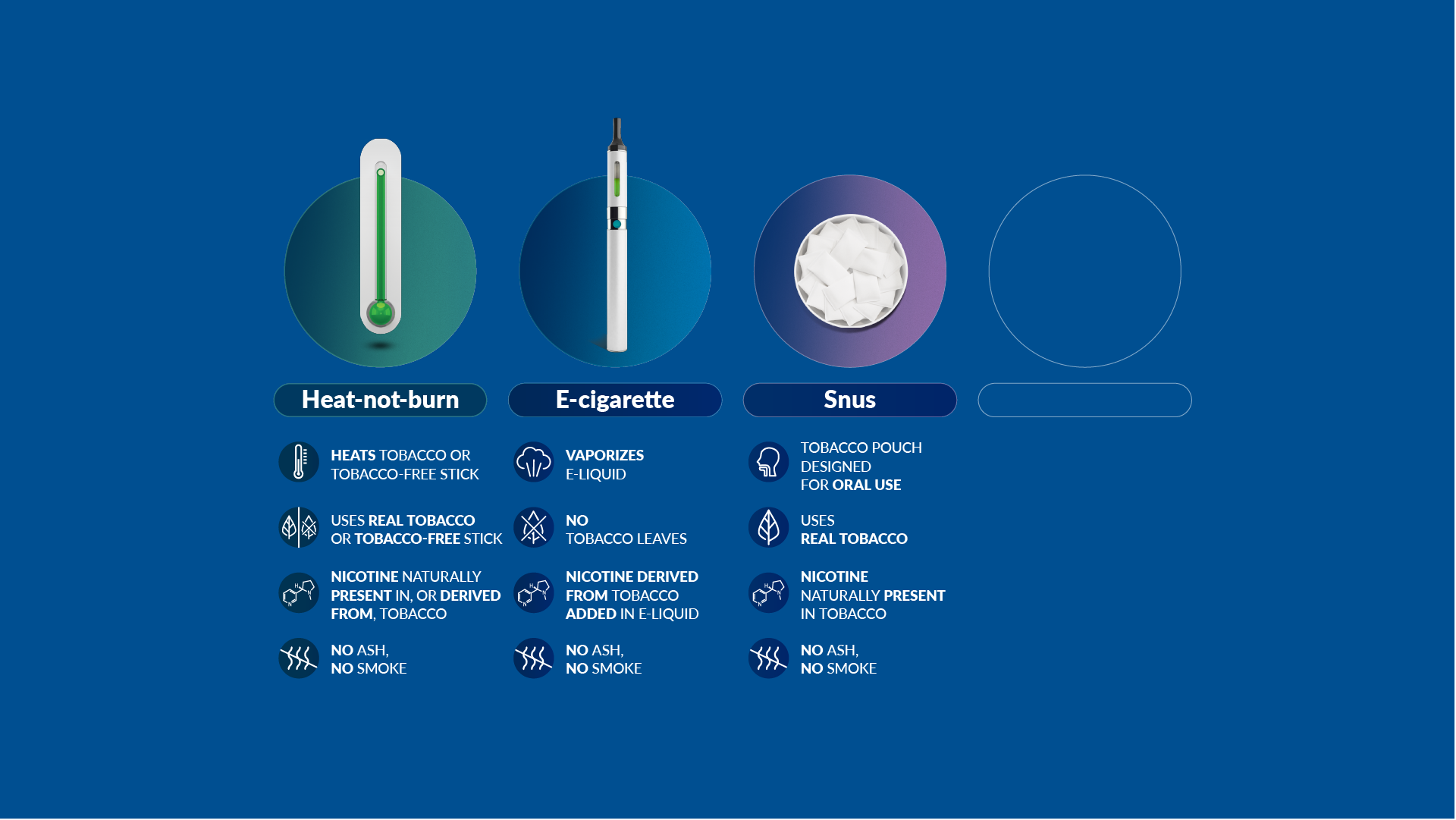
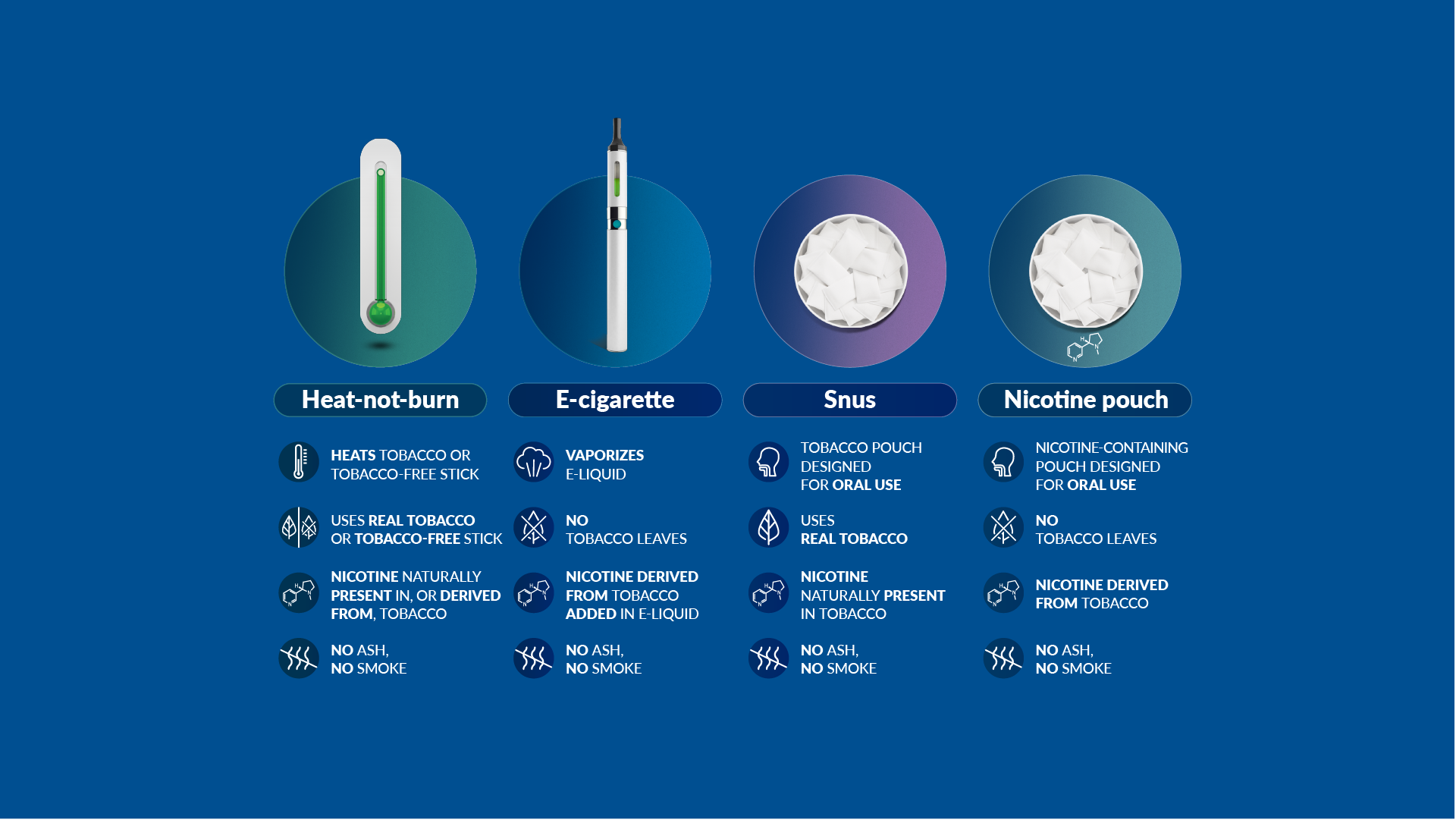
Our scientists have developed a new category of heat-not-burn products, which heat but do not burn the tobacco. We have also developed and commercialized e-cigarettes, which vaporize a liquid solution containing nicotine and flavors. And we now offer nicotine pouches and snus products, which are placed between the gum and the cheek or upper lip.
This portfolio of smoke-free products is actively accelerating the decline of smoking beyond what can be achieved by traditional tobacco control measures alone.
The success of our smoke-free products has transformed PMI
* Smoke-free product net revenues as of Q3 2014.
** Smoke-free business net revenues as of Q4 2025.
In November 2016, our former CEO, André Calantzopoulos, announced that we would build PMI’s future on smoke-free alternatives that are a better choice for adults who would otherwise continue to smoke.
Our scientific results suggested that switching completely to these products would be likely to present less harm than continued smoking.

In July 2020, four years after our initial application, the U.S. FDA authorized the marketing of our flagship smoke-free alternative to cigarettes as a modified risk tobacco product (MRTP). The FDA reviewed over one million pages of documentation we submitted relating to our scientific findings, including aerosol chemistry, non-clinical data, clinical data, consumers studies, and independent studies.
This made it possible to inform American men and women who don’t quit cigarettes that switching completely to our heated tobacco product is a much better choice.
The FDA’s decision provided an important example of how governments and public health organizations can regulate smoke-free alternatives to differentiate them from cigarettes in order to promote the public health.
This decision was consistent with earlier conclusions of other leading regulatory and scientific bodies, including in the U.K., Germany, and the Netherlands, which have found that the product emits lower levels of harmful toxicants.
Back in 2019, Philip Morris International’s affiliate, Swedish Match, became the first company to receive an MRTP authorization from the U.S. FDA for its leading snus brand, which was reauthorized in 2024.
And in January 2025, the FDA authorized all nicotine pouches of Swedish Match’s leading nicotine pouch brand currently marketed in the U.S., under the premarket tobacco application (PMTA) making the brand the first and only authorized nicotine pouch in the US.


.jpg)