Science-based smoke-free products can play a vital role in tobacco harm reduction, as they have the potential to reduce the risks and harms of smoking-related diseases for adults who would otherwise continue to smoke.
Music starts
Voiceover starts speaking:
If tobacco harm reduction works it would be one of the....
Patrick Picavet, Chief Medical Officer, Philip Morris International, sits down and carries on speaking to camera:
...biggest public health impacts that have ever happened and I wanted to be part of that.
The words ‘assessing the health impacts of our products’ is seen on screen.
Lindsay Reese, Publications Manager, Philip Morris International, speaks to camera:
Big tobacco of 50 years ago is very different than what we are today.
Voiceover says:
What we do here is transparent science
Patrick Picavet speaks to camera:
We want to be an active part of the scientific community.
Marina Suvakov, Global Head Safety Surveillance, Philip Morris International, speaks to camera:
We really are focusing on the consumer from start to finish.
Christelle Haziza, Global Head Scientific and Medical Affairs, Philip Morris International, speaks to camera:
It's surprising sometimes to see that scientific people or medical doctors, they are not even aware that the data are existing on smoke-free products.
Emilija Velikovic, Manager Scientific and Medical Affairs, Philip Morris International, speaks to camera:
Typically, people are surprised when they see what type of science we do here at Philip Morris.
Adam Lenart, Manager Population & Public Health Research, Philip Morris International, speaks to camera:
In clinical trials, you can you know possibly look at a relatively small number of people.
Patrick Picavet speaks to camera:
And you collect the data specifically for the study
Adam Lenart speaks to camera:
But with real word data we have the option to look at millions of people
And you know, millions of people well they got to tell you something.
Emilija Velikovic speaks to camera:
That's why we are trying to generate science of high quality to be able to communicate what those differences are between conventional cigarettes and the products that we are developing.
Lindsay Reese talks to camera:
Our team works to take all that science that we've rigorously assessed and analyzed and then bring it into a form that's more accessible.
Voiceover continues:
People don't need to trust us.
They can look at the data evaluate for themselves and see what conclusions they would draw.
Marina Suvakov speaks to camera:
There is nothing that we're doing that we keep for ourselves. Everything is shared and I think that leads to trust over time.
Patrick Picavet speaks to camera:
It's not about creating another addictive product
It is about you know getting people away from smoking combustible cigarettes today.
Marina Suvakov speaks to camera:
The end goal for us is to no longer sell cigarettes and make sure that our consumers have better alternatives.
Christelle Haziza speaks to camera:
I would love regulators to act and to help us to make a cigarette obsolete.
Lindsay Reese talks to camera:
I'm young enough that I think I will be here to see the last cigarette roll off the line at Philip Morris.
Patrick Picavet speaks to camera:
This ability to influence so many people in one go, this is a once in a lifetime, you know, opportunity and frankly you don't get it, you know, that often in your life.
Philip Morris International’s logo is seen on screen.
The words delivering a smoke-free future appear on screen.
As part of a rigorous assessment of the potential of smoke-free products in realizing this, clinical studies and real-world data both have an important role:
- Clinical studies can assess the potential health benefits to adult smokers who switch to our smoke-free products (clinical evidence).
- Real-world data can be analyzed to assess potential benefits of smoke-free products at both an individual and population level (real-world evidence).
It’s vital to understand clinical studies and clinical evidence, as well as real-world data and real-world evidence, how they differ, where the data comes from, and what conclusions can be drawn from them.
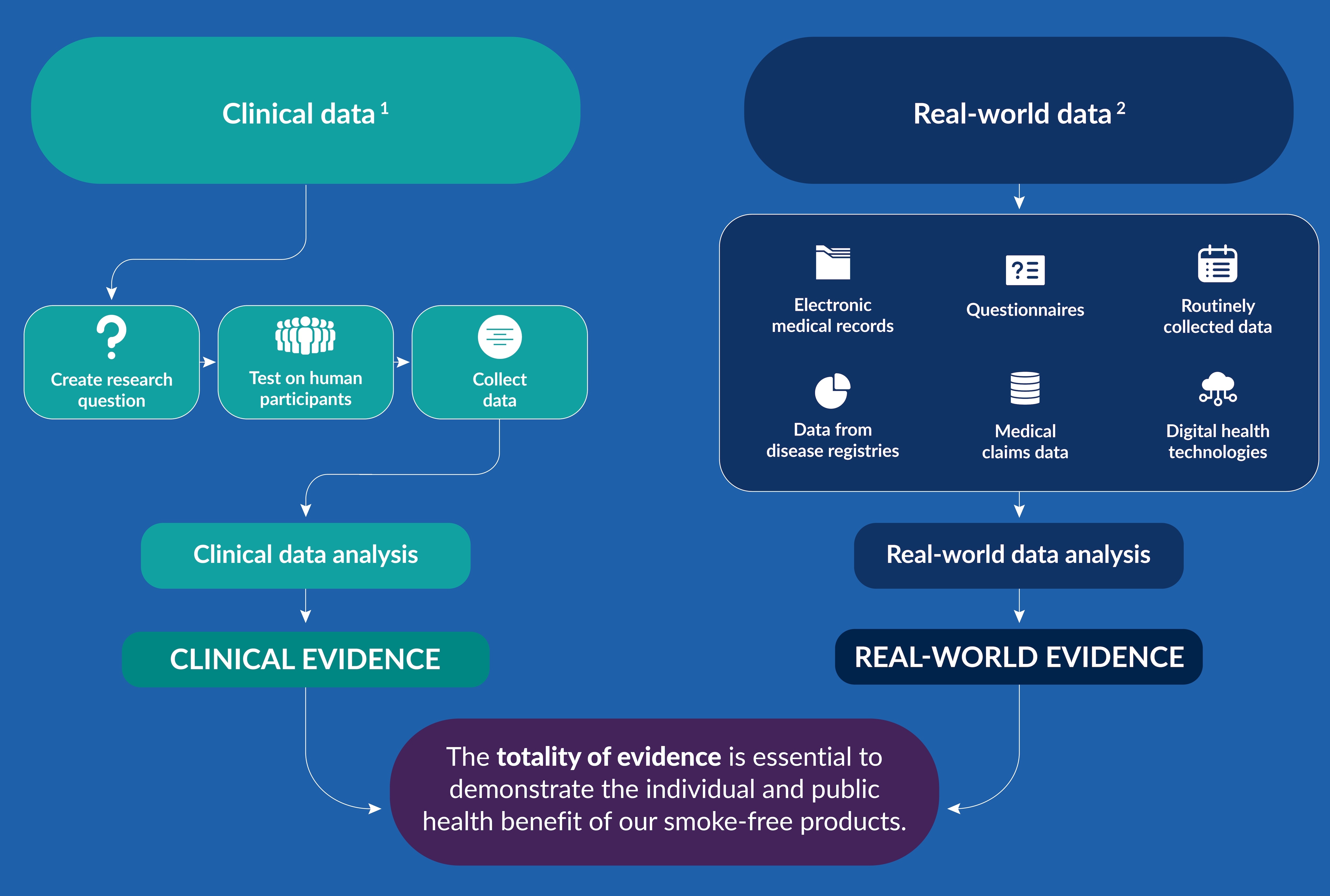
1 Clinical data comes from randomized, controlled clinical studies that assign human participants to one or more products to assess prospectively specific potential health outcomes.
2 Real-world data relates to the health status of and/or delivery of health care to the patient, collected from a variety of sources, such as electronic health records or medical claims data.
Ultimately, Philip Morris International (PMI) aims to use clinical studies and real-world data to generate evidence which can inform the company about the risk-reduction potential of smoke-free products for adult smokers who would otherwise continue to smoke—both on an individual level as well as their potential impact on public health.
Real-world evidence already exists to demonstrate the potential of tobacco harm reduction …
While real-world evidence does not prove a causal relationship, potential trends can be observed, which should provide a better understanding of the potential of tobacco harm reduction on public health.
For example:
- In Denmark, metrics on morbidity (being ill or unhealthy) and mortality due to smoking are about 40 percent higher for females and 60 percent higher for males than in Sweden, a similar country in terms of diet, cultural habits, and demography. 1
Why this difference? It is worth noting that Denmark banned a smoke-free alternative to smoking (snus) in 1992 under EU law, while snus remained available in Sweden. - In Japan, cigarettes sales have declined at a higher rate since the introduction of heated tobacco products in 2015.
… and we are planning clinical and real-world studies to further define the harm reduction potential of our smoke-free products.
These would collect data from adult smokers, people who quit smoking and nicotine products altogether, and users of smoke-free products. The table below provides an overview:
Future evidence generation for major smoking-related diseases
PMI’s roadmap
How we will test the relative risk reduction of our smoke-free products
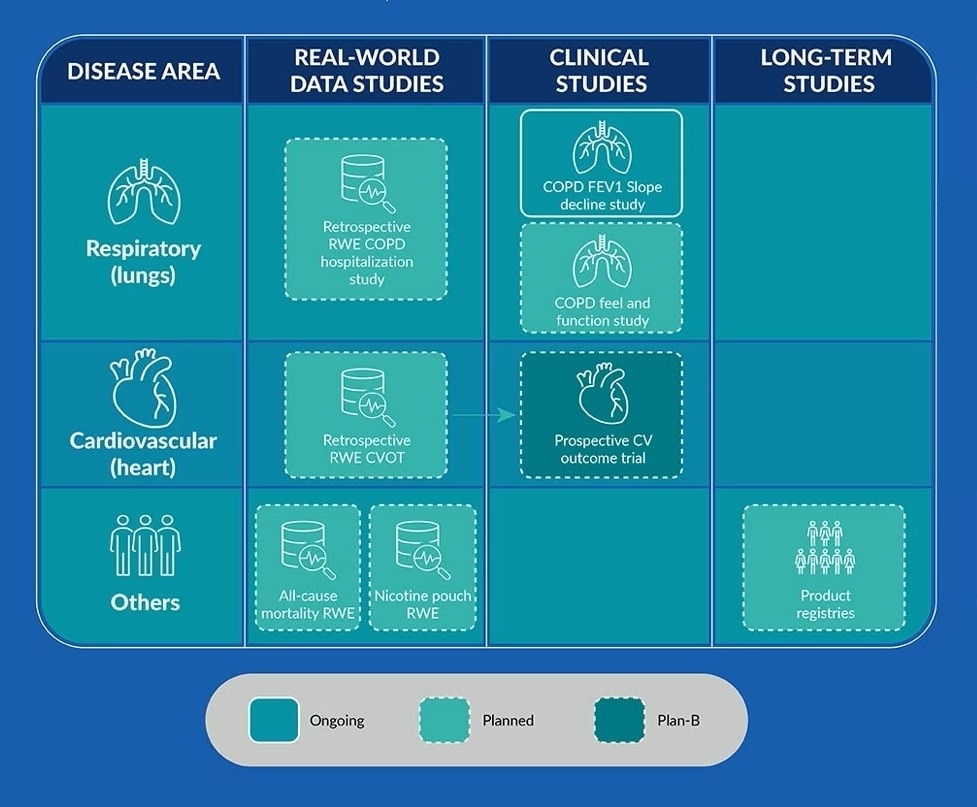
RWE: Real-world evidence. COPD: Chronic obstructive pulmonary disease. CVOT: Cardiovascular outcome trial. FEV: Forced expirator volume (air that can be exhaled in a period of time—a major parameter in obstructive airways disease). CV: Cardiovascular. Product registries: In some markets, consumers could choose to register their smoke-free product and share specific health details on an ongoing basis, allowing us to measure health harm reduction.
PMI’s strategy
How we will plan our studies to show the harm-reduction potential of our smoke-free products
- Healthcare utilization, e.g. hospitalization
- Demonstrate direct clinical benefit
- Feel/symptoms
- Function/activity
- Survival/mortality





