Our studies on our most advanced smoke-free alternative product, the Tobacco Heating System (THS)1, are progressing rapidly. They support the following findings (when used as intended):
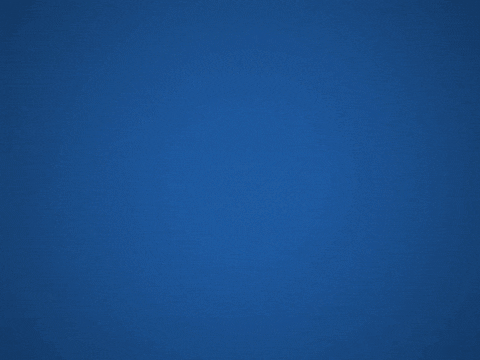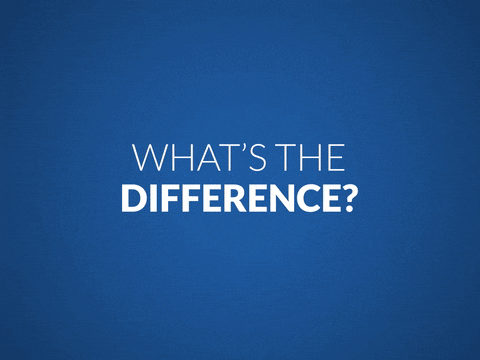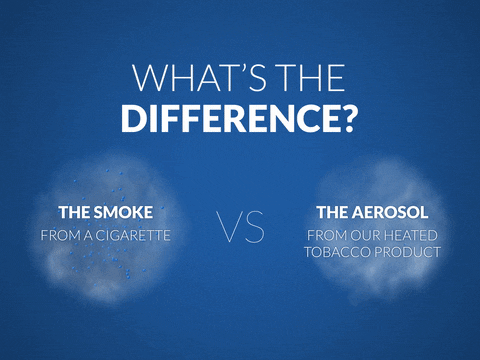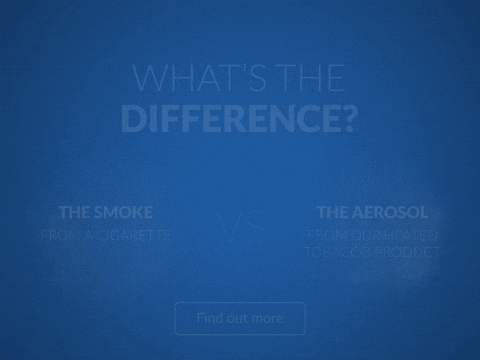
- The THS generates no combustion and no smoke.
- According to laboratory tests, the THS aerosol has an average of 95 percent lower levels of harmful and potentially harmful constituents (HPHCs) and is less toxic than cigarette smoke.
- According to indoor air quality tests, the THS aerosol does not adversely affect indoor air quality. The THS is also not a source of second-hand smoke.
- A 90-day study in the United States and also one 90-day study in Japan reported that adult smokers who switched completely to the THS reduced their exposure to selected HPHCs (based on the measurements of biomarkers of exposure). The reduced levels of biomarkers of exposure were comparable with those observed in people who quit smoking for the duration of the studies. Of course, the best choice is always to quit smoking and nicotine completely.
- Clinical findings from a six-month study on biomarkers of potential harm indicate that switching completely to the THS may have a positive impact on adult smokers’ health.
- Our research shows negligible interest in the THS among people who have never smoked or who have quit smoking.
- 70 percent of adult smokers who switch to the THS use it exclusively or together with other smoke-free products.
- The THS aerosol discolors teeth significantly less than cigarette smoke.
The totality of our studies to date indicates that the THS, while not risk-free, is likely to present less risk of harm compared to continued smoking for adult smokers who switch to it completely.
1 The THS is marketed as IQOS.
At PMI, we are open to everyone—from academic investigators to individuals who want to replicate our findings and look at the data, analyze it, or even do part of the study themselves.
Dr. Badrul Chowdhury
Chief Life Sciences Officer, Philip Morris International
Visit our PMI Science website for more information on our findings, and to learn more about our latest science and technology updates.
What steps are we taking to independently verify our scientific data?
We are committed to seeking independent verification of the scientific data we have generated on our reduced-risk products (RRPs).* This includes:
- Publishing over 500 peer-reviewed publications and book chapters related to our smoke-free products since 2008
- Conducting an in-depth analysis of study reports by independent experts