| INTEGRATED REPORT 2019 |
Experts and many regulatory bodies, such as the U.S. FDA, agree that the primary cause of smoking-related diseases is not nicotine, but the inhalation of harmful and potentially harmful constituents formed as a result of burning tobacco.1 We are therefore developing a portfolio of products that deliver nicotine without combustion – smoke-free products.
Why it is important to us and our stakeholders
Cigarette smoking is the most dangerous form of tobacco use. It causes several serious diseases, including cardiovascular disease, lung cancer, and chronic obstructive pulmonary disease. The best way to avoid the harms of smoking is never to start or, for those who do smoke, to quit. However, according to WHO forecasts, there will still be more than 1 billion smokers by the year 2025. For these people, alternative products that significantly reduce the risk of disease compared with continued smoking are fundamental. When properly regulated, they can complement existing regulatory efforts aimed at reducing smoking prevalence.
PMI is investing in the development and rigorous scientific assessment of a portfolio of potentially reduced-risk alternatives to cigarette smoking. Our approach is based on the acknowledgment that new products will benefit public health if they meet three conditions: First, the totality of evidence must demonstrate that a product has the potential to significantly reduce risk in the long-term, compared with continued smoking. Second, adult smokers must consider them acceptable alternatives to cigarettes and be willing to switch to them exclusively. And third, their use by unintended audiences – youth, never smokers and former smokers – should be minimized.
Achieving our aims
Science and technology are essential in devising solutions for bringing an end to cigarette smoking. The principal source of cigarette-related diseases is known: It is the burning process that creates the vast majority of the harmful chemicals that are the primary causes of smoking-related diseases. In our smoke-free tobacco products, we are able to precisely control temperatures so that they release nicotine and flavors but do not reach the temperature necessary for combustion to occur. By avoiding combustion, we reduce or eliminate the formation of harmful and potentially harmful chemicals (HPHCs).
The toxicological assessment of smoke-free products is guided by two fundamental principles. The first principle is derived from epidemiology: Smoking-related harm and disease are directly caused by long-term exposure to the toxicants found in cigarette smoke. The best way to avoid these harms is never to start smoking. For men and women who smoke, cessation has been demonstrated to lead to reduced harm and risk of tobacco-related disease by eliminating exposure to cigarette smoke toxicants.
Complete, long-term cessation is the maximum risk-reduction that a smoker can achieve, and hence is the “gold standard” for the assessment of smoke-free products. The second principle is derived from toxicology: The degree of exposure to toxicants determines the nature and degree of adverse health effects. For exposure to take place, toxicants need to be present – meaning that they are emitted by the product or process of use. A product with significantly reduced toxic emissions compared with cigarettes has the potential to significantly reduce exposure to toxicants, which in turn will lead to a reduction in adverse health effects. In line with those two principles, our toxicological assessment program aims to compare the health outcome of switching to smoke-free products with ongoing smoking and with cessation. Smoke-free products are not risk-free, and they contain nicotine, which is addictive. The way we assess the risk of using them is always in comparison with cigarette smoking or cessation.
To demonstrate that switching to our smoke-free products results in reduced exposure to toxicants, which can or does reduce the risk of disease compared with continued cigarette smoking, we are following a rigorous scientific assessment program aligned with the U.S. FDA’s draft guidance on modified risk tobacco products (learn more about how we conduct our R&D). Our assessment program also aims to guard against the use of our smoke-free products by unintended audiences, such as former smokers, never smokers, and youth.
Our two main R&D centers, located in Switzerland and Singapore, employ hundreds of scientists, engineers, and other experts to work on the development and assessment of smoke-free products. This work is headed by our Chief Product Officer, Chief Life Science Officer, and Chief Consumer Officer, who are members of PMI’s Company Management. In 2019, 98 percent of our R&D expenditure was dedicated to smoke-free products. This includes costs related to clinical studies, the development of machinery and prototypes, and product acceptability testing. The remaining 2 percent of our total R&D expenditures largely relate to regulatory compliance requirements for our combustible tobacco products. The development and assessment of smoke-free products involve a network of organizations with which we partner worldwide, including start-ups, laboratories, and universities (see the Swiss example in our case study). It is the responsibility of any manufacturer to assess and demonstrate that switching completely to a novel tobacco and nicotine product has the potential to reduce the risk of harm compared with continued smoking. To ensure trust, this assessment must be independently verified. PMI has adopted both passive and proactive approaches to verification. Passive approaches include independently funded studies without interaction from PMI, as well as the in-depth review and inspection of PMI’s R&D and manufacturing processes, study documentation, data, and premises by regulatory agencies upon regulatory submissions. Just as with our own research, it is essential that any independent study be conducted with the appropriate degree of scientific rigor. The methodologies and equipment used should be fit-for-purpose and validated, and the experimental results should be interpreted and reported in an accurate and non-misleading manner.
To complement these passive approaches, we have developed a strategy to build confidence in our scientific methods and results by taking several proactive steps, including the periodic inspection and renewal of ISO 17025 for testing and calibration laboratories and Good Laboratory Practices (GLP) accreditations by national authorities; publication of our methods and results in peer-reviewed scientific literature or on platforms such as ClinicalTrials.gov; crowdsourced verification of our methods and study results through the sbv IMPROVER platform; and proactive sharing of data to enable analysis by independent third parties through the INTERVALS platform. Read more on the transparency of our R&D here.
It is clear that long-term epidemiological data are needed to accurately quantify the overall disease risk reduction effect associated with switching from cigarette smoking to the use of smoke-free products, as well as to evaluate the excess disease-risk associated with the use of these products. Generating epidemiological evidence requires that smoke-free products be available in the market for many years. The products must be used exclusively by a large portion of adult smokers who have fully switched for at least a decade. This renders epidemiological studies impracticable in a premarket setting or in the early phase of market introduction.
Smoke-free products timeline
PMI has been working on innovative, noncombustible tobacco products to replace cigarettes for decades. After years of research and two unsuccessful commercialization attempts, we launched IQOS, a heat-not-burn product and our main smoke-free product, in 2015. As of the end of 2019, the product was available in 52 markets, and we estimate that approximately 9.7 million adults had stopped smoking and switched to it.
Scientific challenges in the assessment of smoke-free products
Quantifying precisely the disease risk-reduction potential of smoke-free products before, or soon after, they are introduced into the market comes with scientific challenges.
The first important challenge is that most smoking-related diseases generally occur after decades of smoking. Additionally, the reduction in excess risk upon smoking cessation – and therefore also upon switching to smoke-free products – also takes years. This is complicated even further because most smoking-related diseases are also affected by other important factors such as diet and lifestyle. Together all of this makes the measurement of health outcomes after switching difficult – if not impossible – in the short-term.
The second challenge is that smoking affects several organ systems and many biological mechanisms. As a result, no single biomarker can, on its own, coverall of the smoking-related diseases. Therefore, several biomarkers need to be considered together, to strengthen the evidentiary basis with coherent, mutually supportive data that cover the multifaceted impact of cigarette smoking. Scientists and regulators have not yet reached consensus on the range of biomarkers that should be used to assess the effects of smoking, smoking cessation or switching to smoke-free products.
Considering these two challenges in particular – the complexity and multifaceted nature of smoking-related diseases and the long-term nature of measuring disease – a totality of evidence approach is fitting in the short term to assess the disease risk reduction potential of smoke-free products. This approach takes evidence from different studies to cover all of the steps in disease development from the emission of toxicants all the way to overt disease. For IQOS, the totality of evidence generated by PMI brings together extensive aerosol chemistry data, nonclinical studies (18 studies, including animal models of disease covering the major smoking-related diseases), and clinical studies (10 studies with thousands of participants with up to six months of exposure). The strength of this evidence comes from the coherence and consistency of the results across the different studies, endpoints, disease pathways, and biological systems all pointing in the direction of risk reduction. It is the totality of the evidence that allows us to conclude that IQOS reduces the risk of smoking-related disease.
U.S. vaping investigation

In August 2019, the U.S. Centers for Disease Control and Prevention (CDC) and the FDA started an investigation into almost 3,000 reported cases of respiratory illnesses thought to be connected to vaping. The U.S. CDC termed the illness “e-cigarette or vaping product use-associated lung injury” (EVALI) and, sadly, there were 68 confirmed deaths according to their last and final report from February 18, 2020. CDC’s investigation concluded that vitamin E acetate was strongly linked to the EVALI outbreak.
This chemical appears to have been added to tetrahydrocannabinol (THC)-containing vaping products – particularly those from illicit sources – which played a major role in the outbreak. Over the latter half of 2019, there was significant – often inaccurate – news coverage of EVALI, not just in the U.S. but also worldwide. These news reports often incorrectly conflated EVALI with the use of legal, regulated e-cigarette products and were seized upon by special interest groups opposed to tobacco harm reduction.
The unfortunate outcome has been confusion among smokers and e-cigarette users, resulting in a rise in the incorrect perception that e-cigarettes are equally or more harmful than cigarettes. More worryingly, there have also been reports that smokers who had switched to e-cigarettes are turning back to cigarettes, particularly those in older age groups. Once the CDC’s conclusions were available in February 2020 – making the connection between EVALI and illicit products – the damage had already been done, to the detriment of men and women who smoke.
When manufactured according to proper safety and quality rules and responsibly commercialized, smoke-free products like e-cigarettes, while not safe, have the potential to reduce the risk of tobacco-related harm for adult smokers who switch completely from combustible cigarettes. Their development, assessment, and use by adult smokers who otherwise would continue to smoke should be encouraged in the interests of public health. They should also be subject to regulation and governmental oversight. It is particularly important that regulation gives consumers confidence in the quality and safety of the products they use, and that manufacturers develop evidence that their products are better than cigarettes for adult users and for the public health overall.
Our heat-not-burn IQOS product
What is it and how does it work?
Our main smoke-free product, IQOS, is a battery-powered heat-not-burn product, which heats the tobacco to produce a nicotine-containing aerosol that is inhaled by the user without combustion of the tobacco. IQOS works as a tobacco-heating system and is composed of three main components: a heated tobacco unit, a holder, and a charger. The electronically heated tobacco unit is a novel product containing specially processed tobacco and two filter sections. The unit has been designed specifically and exclusively for use with the holder (the heating device). It contains a processed tobacco plug designed to be heated but not burned. It is made of tobacco leaves, which are ground and reconstituted into tobacco sheets, called “cast-leaf.” These sheets are then crimped and made into a tobacco plug. The user inserts the heated tobacco unit into the holder. Once activated, the holder heats the tobacco via an electronically controlled heating blade. The holder stores sufficient energy to permit the use of one or two heated tobacco units, depending on the IQOS model. Each tobacco unit provides a maximum of 14 puffs or around six minutes of consumption, whichever comes first. The holder contains a small battery, which needs to be recharged by inserting it into the charger, which in turn can be recharged using a household power socket. The integrated version of the product, IQOS Multi, combines the holder and charger and allows for the use of up to 10 heated tobacco units before it is necessary to recharge the battery. We continue to enhance our IQOS product, driven by consumer insights and supported by scientific substantiation. In September 2019, we launched the latest generation of the device, IQOS DUO, with new technology that allows for fast charging and two consecutive uses without recharging the holder.
The difference between heated tobacco products and e-cigarettes
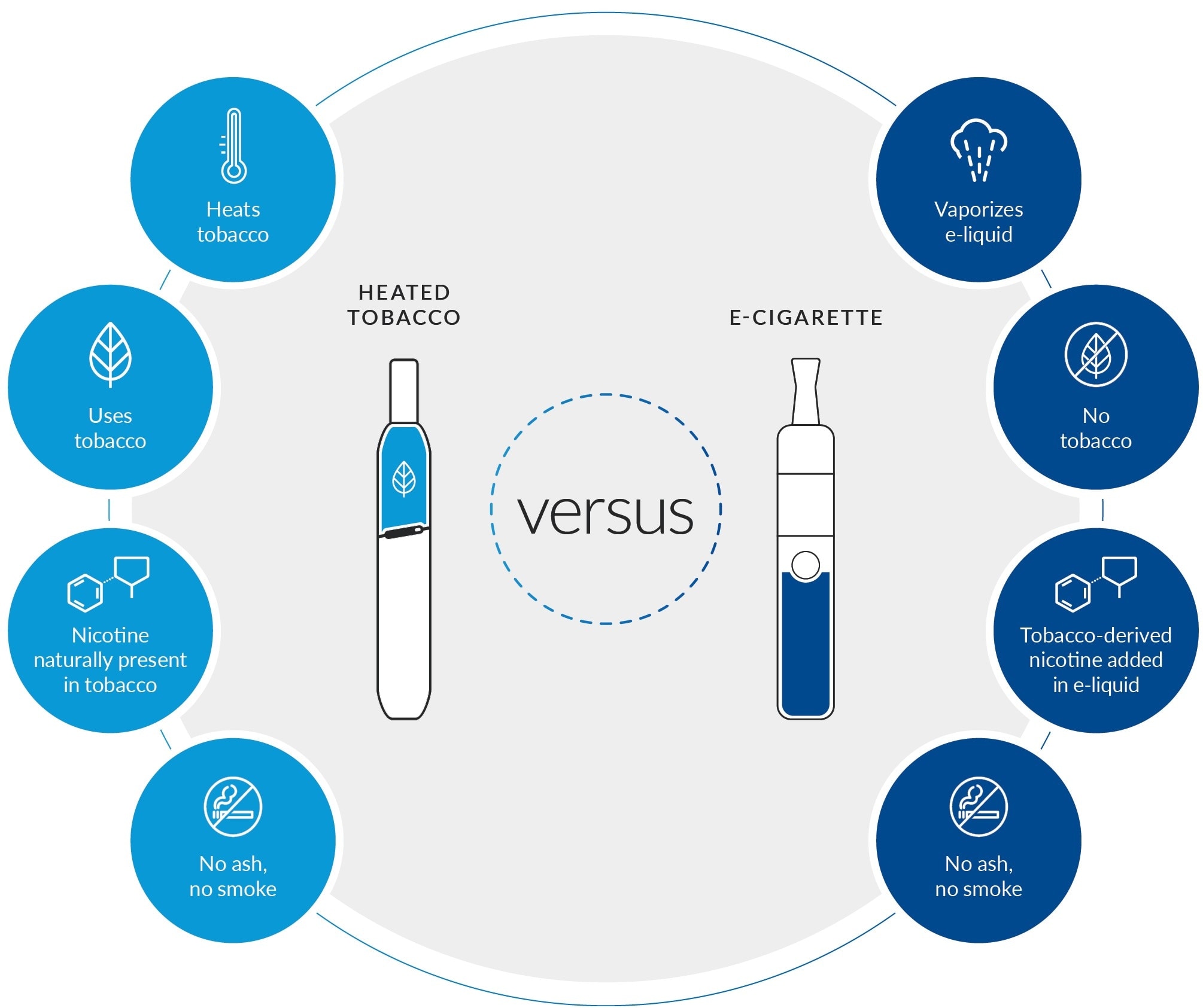
How does it reduce risk?
IQOS reduces the risk to health, compared with cigarette smoking, by avoiding burning tobacco. Decades of epidemiological data have demonstrated that the development of smoking-related diseases is triggered by the chronic inhalation of the harmful and potentially harmful chemicals (HPHCs) found in cigarette smoke. When a smoker lights a cigarette, it starts a high-temperature reaction, from 600 to 900 degrees Celsius, known as burning or combustion, which releases HPHCs. When IQOS is used, the device constantly monitors and controls the temperature of the tobacco so that it stays below 350 degrees Celsius. As a result, the nicotine-containing vapor the consumer inhales contains significantly lower levels of HPHCs than cigarettes. We have scientifically substantiated that during the operation of IQOS, no combustion occurs, and the aerosol generated has on average 95 percent lower levels of HPHCs than found in reference cigarette smoke.
The different parts of an IQOS 3 device
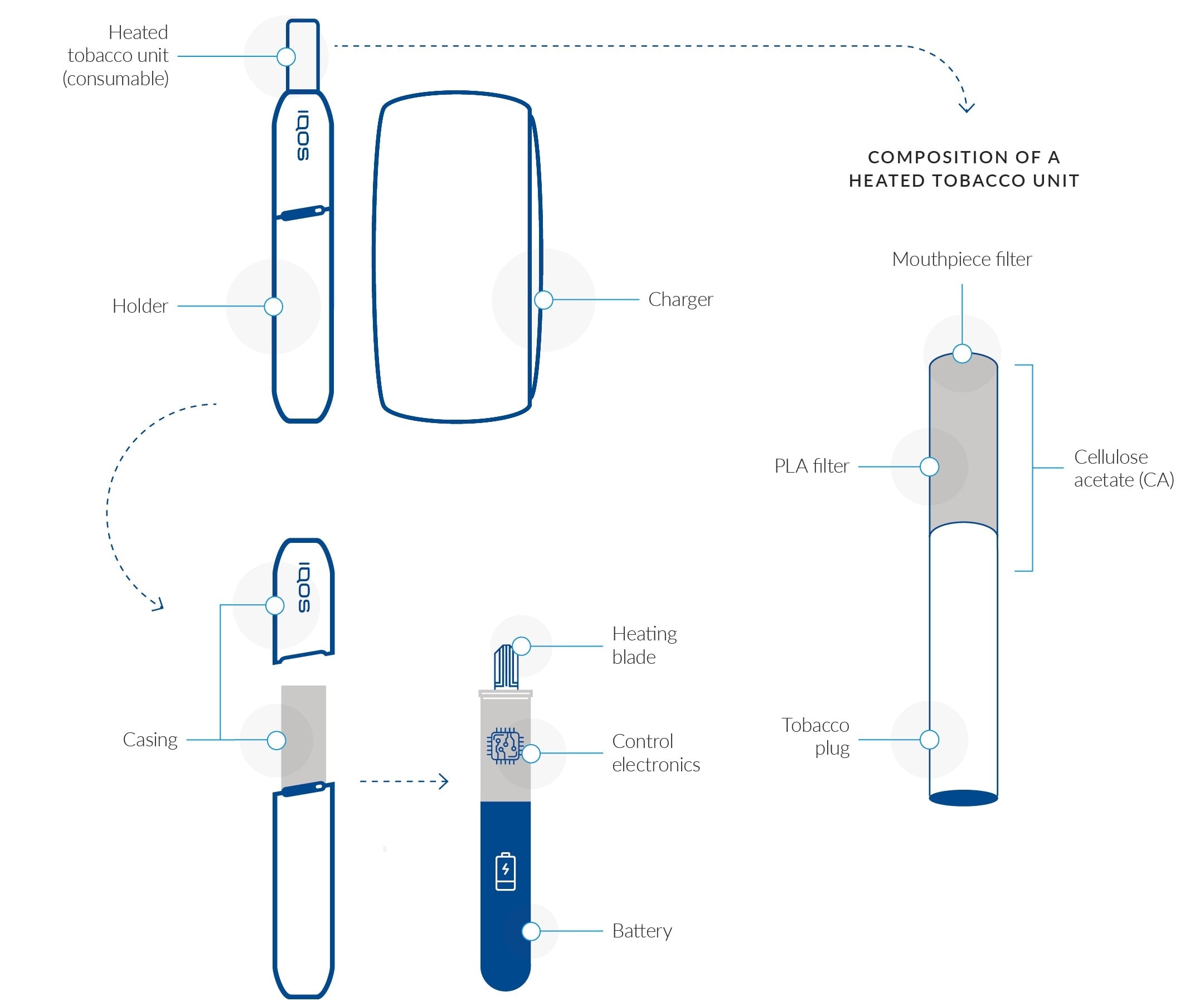
What scientific evidence have we gathered to date?
IQOS is the most developed and most thoroughly assessed of our smoke-free platforms. We have accumulated extensive clinical and nonclinical data to support its potential to reduce the risk of developing smoking-related diseases compared with continued cigarette smoking.
As a first step, we compared the chemical composition of the IQOS aerosol with that of the smoke from a cigarette, using standardized and validated analytical methods to quantify the most important HPHCs known to be carcinogens or respiratory or cardiovascular toxicants, or to be involved in other toxic effects. We found that HPHCs are reduced on average by 95 percent compared with the reference cigarette's smoke – importantly, the carcinogens are reduced on average by more than 95 percent. Notably, nitrogen oxides and carbon monoxide, two important combustion markers, are reduced by over 96 percent. We conducted extensive chemical characterization of the IQOS aerosol. Our state-of-the-art untargeted screening has allowed us to characterize approximately 99.8 percent of the total aerosol mass, and we have identified all constituents present at levels ≥100 ng/stick. These analyses showed that there were over seven times fewer constituents in IQOS aerosol compared with reference cigarette smoke. As the U.S. FDA put it when they authorized a version of IQOS for sale in the U.S., they are “present at very low levels” and “do not raise significant concerns from a public health perspective.”2 We also conducted indoor air chemistry and quality studies, demonstrating that the use of IQOS does not negatively impact indoor air quality.
We then conducted toxicological studies for IQOS, both in vitro and in vivo, to determine whether the reduced formation of HPHCs in the aerosol leads to reduced toxicity in laboratory models. Results from the in vitro studies showed that the IQOS aerosol is significantly less cytotoxic and genotoxic than the smoke from cigarettes. The in vivo study found that, compared with cigarette smoke, the reduced exposure to toxicants achieved with the IQOS aerosol leads to a significantly reduced lung inflammation and respiratory toxicity.
In the following stage, we conducted several systems toxicology studies to assess the disease-relevant biological mechanisms affected by exposure to toxicants, using human-derived in vitro cell cultures and organotypic tissue cultures. These studies showed that, compared with the reference cigarette smoke, the IQOS aerosol has a significantly reduced impact on key mechanisms involved in the development of respiratory and cardiovascular diseases. In a systems toxicology study conducted in an animal model of disease, we observed that switching to the IQOS aerosol following two months of cigarette smoke exposure reduces the development of both atherosclerosis and emphysema in a manner similar to smoking cessation. Recently, we completed an in vivo study to assess the potential of the IQOS aerosol to reduce the risk of lung inflammation, emphysema, and cancer compared with cigarette smoke; we expect to share the findings soon.
We conducted more than 10 clinical studies on IQOS involving thousands of participants and up to six months of exposure. For example, we conducted four clinical studies, each involving 160 participants, and an exposure period lasting five days to three months, to assess relative exposure to toxicants when switching to IQOS compared with continued cigarette smoking. The first two studies – incorporating a five-day exposure, in confinement – were conducted in Europe and Japan. The subsequent two studies extended over three months – a five-day exposure period followed by an ambulatory period of 85 days – and were conducted using IQOS with menthol version heated tobacco units in Japan and the U.S. The ambulatory study period was intended to assess whether reductions in exposure observed in a confined setting were sustained under more “real world” conditions, where confounding factors such as environment, diet, passive smoking, and the use of combustible cigarettes in combination with IQOS (“dual use”) could influence the levels of exposure to HPHCs. All four studies showed a significant reduction (ranging from 47 percent to 96 percent relative to continuing cigarette smoking) in the 15 biomarkers of exposure in adult smokers who switched to IQOS. In addition, we conducted an exposure response study to measure clinical risk markers when adult smokers switch to IQOS for a six-month period. The results of this study show that all eight co-primary clinical risk markers display favorable changes upon switching to IQOS and that favorable changes in five out of eight markers are statistically significant.
In 2019, we launched long-term assessment tools to find out how consumers understand the reduced risk associated with smoke-free products and how that understanding influences their choices. The new survey tools are intended to measure what people think about and how they use the products across a set of categories, such as perceived risk, perceived dependence, and functioning and well-being. Results of the assessment are available to the public in an article on F1000Research,3 and its peer review is ongoing.

IQOS is the most developed and most thoroughly assessed of our smoke-free products
What are the external findings to date?
Our scientific results are supported by a growing body of independent research. Several government agencies are taking an interest in validating the available evidence or conducting research of their own. For the period of January 2019 to February 2020 alone, more than 60 third-party peer-reviewed publications and systematic reviews have been published on heated tobacco products. In addition to studies on aerosol chemistry and toxicology, the first independent clinical and post-market studies have started to emerge. In general, these studies confirm PMI’s results, although some have contradictory interpretations of similar results to ours (e.g., using fresh air as the comparator instead of cigarette smoke), methodological differences (e.g., studies that do not follow OECD guidelines), or overstated conclusions. The list of independent studies published around PMI’s smoke-free products and/or our methods and results as of December 19, 2019 is available on our PMIScience website
In 2019, a version of the IQOS system became the first electronic heat-not-burn product to be authorized for sale in the U.S., pursuant to the 2009 law that empowers the FDA to regulate tobacco products, including through oversight of innovative products. The agency stated: “Following a rigorous science-based review through the premarket tobacco product application (PMTA) pathway, the agency determined that authorizing these products for the U.S. market is appropriate for the protection of the public health because, among several key considerations, the products produce fewer or lower levels of some toxins than combustible cigarettes. (...) While today’s action permits the tobacco products to be sold in the U.S., it does not mean these products are safe or ‘FDA approved.’” The FDA published a detailed report describing its assessment and conclusions, including results on aerosol chemistry, toxicology, and unintended use. The agency found that “the aerosol produced by the IQOS Tobacco Heating System contains fewer toxic chemicals than cigarette smoke, and many of the toxins identified are present at lower levels than in cigarette smoke” and that “Available data, while limited, also indicate that few non-tobacco users would be likely to choose to start using IQOS, including youth."4
The authorization is subject to strict marketing, reporting, and other requirements and is not a guarantee that the product will remain authorized, particularly if there is a significant uptake in youth initiation. The FDA will monitor the marketing of the product. The FDA is continuing its scientific review of PMI’s MRTP applications, which were submitted for the same products on December 5, 2016. An MRTP order from the FDA would allow a tobacco product to be marketed to adult smokers in the U.S. with messages stating that switching completely from cigarettes to IQOS (1) reduces exposure to certain chemicals, and/or (2) that the product is less harmful than another tobacco product or would reduce the risk of tobacco-related disease.

Our e-vapor product
What is it and how does it work?
Our second smoke-free product, IQOS MESH, is an e-vapor product (or e-cigarette), which is a battery-powered device producing inhalable vapor from a liquid solution containing nicotine and flavors. We plan to launch the next generation of IQOS MESH, which will be launched under the brand name IQOS VEEV. This product is designed to maximize acceptance of adult smokers who would otherwise continue to smoke and facilitate complete switching in a manner similar to our heat-not-burn product. IQOS VEEV is composed of a device and a disposable cartridge. The device holds the battery and the puff sensor, while the cartridge holds the heating element and the e-liquid. IQOS VEEV is based on a new heating technology in the e-vapor product category. When the device is switched on, puffing draws air through the bottom of the cartridge, activating the heater. To generate the vapor, the product uses a metallic mesh punctured with tiny holes, which heats e-liquid contained in the cartridge (commercialized under the VEEV brand). The liquid contains flavors and nicotine, the latter being extracted from tobacco leaves. IQOS VEEV uses closed-system e-liquid cartridges to protect against tampering and liquid leakage. Unlike typical e-vapor product cartridges, VEEV cartridges are manufactured, assembled, pre-filled, and pre-sealed in a fully automated process, to ensure product consistency. This occurs within our European production facilities. Each cartridge contains a heater, which eliminates the need to manually replace it, and the liquid in the cartridge is sufficient for the device to be used several times for a given cartridge. IQOS VEEV also features puff-activated heating and a low-liquid level detection system that ensures the consistency and quality of the vapor generated and inhaled.
How does it reduce risk?
In e-vapor products, the liquid is heated to form an aerosol, which is inhaled through the mouthpiece. In contrast with cigarettes, e-vapor products deliver nicotine without the smoke constituents that arise from the combustion of tobacco. Until recently most e-vapor products used a “coil and wick” system to heat the nicotine-containing solution. The wick draws the liquid onto a coil-heating element to heat the liquid and create an aerosol. In these systems, the temperature of the heater can vary significantly depending on how strongly the user puffs on the e-cigarette. Several studies have shown that puffing on an e-cigarette when the liquid level is low results in “dry puffs,” which can significantly increase exposure to certain toxicants – especially formaldehyde. In IQOS MESH and IQOS VEEV, the wick is eliminated, and the coil is replaced by a metal mesh. When the liquid is introduced to the heater, the increased surface area provided by the mesh material helps heat the liquid in a more consistent way than it occurs with current-generation coil and wick systems. The software ensures that the temperature of the heater is stable and does not vary based on the puff strength or puff-to-puff. The low-liquid detection system cuts off the power supplied to the mesh heater once the level of the liquid has dropped below a certain level, eliminating dry puffs.

In IQOS MESH and IQOS VEEV, the wick is eliminated, and the coil is replaced by a metal mesh
Next steps
We will continue to invest in scientific research to develop a portfolio of ever-better, affordable, and acceptable smoke-free products for existing adult smokers – in both developed and developing countries. In addition to continuously improving the two smoke-free product platforms we are currently commercializing, we are continuing R&D work on our other two product platforms. Our R&D work, and its associated innovations, has resulted in 5,800 patents granted for smoke-free technologies as of the end of 2019. As of this year, we are introducing a new metric that tracks the number of patents and patent applications published and granted by the IP5 Offices. IP5 is the name given to a forum of the five largest intellectual property offices in the world; the IP5 Offices together handle about 80 percent of the world’s patent applications. The revision to the reported metrics reflects PMI’s refined international patent filing strategy, by which it is ensuring that it is focusing its resources on enhancing patent protection for its innovations in key jurisdictions. The refined strategic geographic footprint of its patent portfolio optimizes IP value for money, reducing the average unit cost for protecting its innovations internationally, and freeing up resources to enhance the patent portfolio in key jurisdictions.
1Source: FDA
2Source: FDA (page 41)
3Reference: https://f1000research.com/articles/8-214
4Source: FDA
This online supplement to our integrated report should be read in conjunction with PMI’s Integrated Report 2019. The information and data presented in this online supplement cover the 2019 calendar year or reflect status at December 31, 2019, worldwide, unless otherwise indicated. Where not specified, data come from PMI estimates. See About this online supplement for more information. Aspirational targets and goals do not constitute financial projections, and achievement of future results is subject to risks, uncertainties and inaccurate assumptions, as outlined in our forward-looking and cautionary statements.







